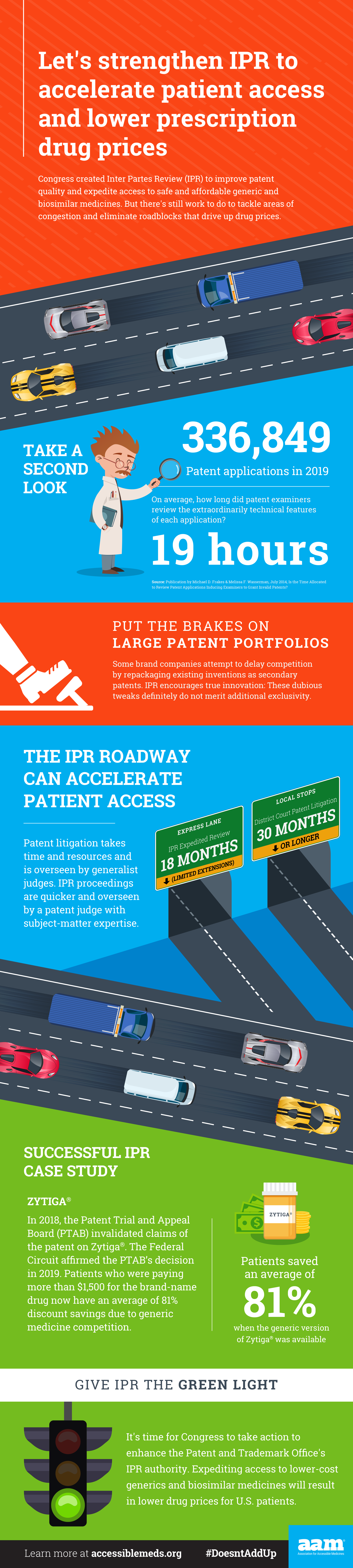
Congress created Inter Partes Review (IPR) to improve patent quality and expedite access to safe and affordable generic and biosimilar medicines. IPR is a process that allows patent experts to take a second look at patents and ensure that every granted patent represents true innovation. This graphic demonstrates how IPR improves patent quality, accelerates competition and makes lower-cost generics and biosimilars accessible to U.S. patients. Learn more in this infographic:
Take action now:
- Read more about this infographic on our blog: Competition Matters. That’s Why We Need to Further Improve IPR.
- Learn more about the campaign #DoesntAddUp
- Share this page to raise awareness about this issue
Issues