EXPLORE INNOVATIONS FROM GENERICS AND BIOSIMILARS
GRx+Biosims™ 2024 is the leading scientific, regulatory, and policy event for the U.S. generics and biosimilars industry. Access the most recent information straight from experts at the Food and Drug Administration, grasp the latest policies shaping the industry, and acquire valuable insights to propel your professional development.
BE INFORMED +
GAIN INSIGHTS
Bench scientists and technical professionals can gain insights from drug regulators to help expedite approvals and speed to market, and learn about the latest innovations and emerging technology. Policy professionals, industry professionals and regulators have the opportunity to network with others in the industry and gain a fuller understanding of how regulations affect the real world. In addition, connect with global influencers, increase your knowledge base to stay on the cutting edge and learn about best practices to enhance your organization’s overall performance.

Why You Should Attend
Network with speakers, attendees, exhibitors and sponsors
Get access to the latest information about U.S. generics and biosimilars
Gain expert insights on implementation of the latest regulations, guidances, case law, current health authority processes and practices and policy
Learn how legislation related to the generics and biosimilars industry can impact patient access to more affordable medicines
Make more informed decisions by better understanding FDA’s expectations and current thinking on processes, practice and policy
Attend this one-stop-shop for all your U.S. generics and biosimilars industry policy, regulatory and scientific needs

Join us for a special session reflecting on the remarkable career of Dr. Janet Woodcock, who recently retired from the U.S. Food and Drug Administration after 38 years of service. Dr. Woodcock been called one of the most powerful and influential drug regulators who has left a historic mark on the pharmaceutical industry and public health.
This session, hosted by Women in Health Policy (WiHP) and powered by the Association for Accessible Medicines (AAM), will dive into Dr. Woodcock’s impactful contributions to the generic and biosimilars industry and her legacy within the FDA. We will discuss her leadership, achievements, and the lasting impact of her work on drug regulation and accessibility.
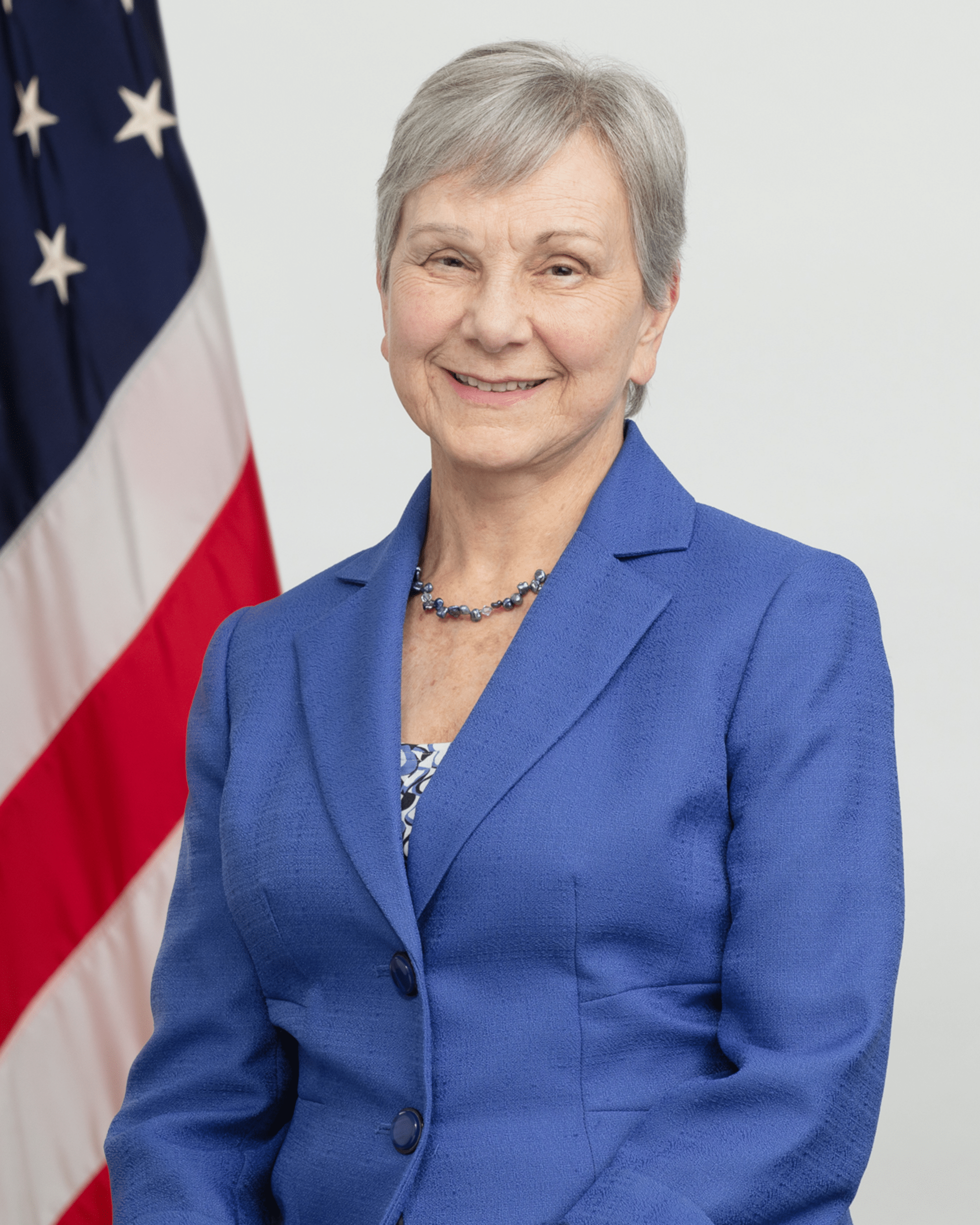
Janet Woodcock, M.D.
Former Principal Deputy Commissioner, FDA
EXPO
Showcase your company at GRx+Biosims 2024. Don’t miss out on this highly interactive and professional business environment in which industry vendors can meet generics and biosimilars industry executives. We offer our exhibitors abundant networking time with industry decision-makers. To learn more, email Aquera Agee.

Sponsorship Opportunities
Raise your company profile by participating in this prestigious event. Included in your sponsorship are custom branded digital assets you can use to promote your sponsorship of the event. You will also gain visibility to your brand through conference advertising prior to and during the event. For more information regarding sponsorship levels and benefits contact Jennifer Soup.
Publication Partners






STAY INFORMED WITH THE MOST RECENT UPDATES
Access up-to-date event details, including information about prominent speakers, sponsorship options, and exhibition opportunities.













GRX+BIOSIMS 2024
SOCIAL MEDIA TOOLKIT
Attending the GRx+Biosims 2024 Conference? Help us spread the word about this outstanding event. We have all kinds of ready-to-use, customizable content that’s quick and easy to share.
Stay up-to-date on the conference by following the event hashtag #GRxBiosims.
Download Coming Soon
SHARE ON X
SHARE ON FACEBOOK
SHARE ON LINKEDIN